
CORONAVIRUS ANTIGEN RAPID TEST
The rapid antigenic test for the detection of Covid-19
The Coronavirus Antigen Rapid test marketed by Diatheva is a rapid immunochromatographic test used for the detection of the nucleocapsid antigen protein from SARS-CoV-2. The test is performed starting with nasopharyngeal swabs from individuals suspected of COVID-19.
The test provides a quick diagnostic reference for COVID-19.
The kit is CE-IVD certified and is intended for professional use only.

What’s the use?
The Coronavirus Antigen Rapid Swab is a rapid test for the detection of the nucleocapsid antigen protein from SARS-CoV-2 and therefore
to identify the ongoing infection in humans.
How does it work?
The rapid antitgenic test for the detection of Covid-19 can be quickly performed without the need for intravenous sampling. Coronavirus Antigen Rapid test is able to return the result in just 15 minutes with the aid of a simple swab and without the need for any accessory equipment.
Whom is it for?
Coronavirus Antigen Rapid test is intended to be used by medical personnel working in hospitals, analysis laboratories and companies. It is particularly suitable for all situations where a it’s necessary a quick analysis of the health status of people who must have access to public and private care facilities and staff working in companies that are preparing to resume their activities.
Selling Points

Ease of use

Quick reading of the result within 15 minutes

No additional equipment needed

Storage at room temperature

High percentages of specificity and sensitivity
How is the test performed?

Use the sterile nasopharyngeal swab provided in the kit, and carefully insert the swab into the patient’s nostril.

Add approximately 10 drops of the extraction buffer to the extraction tube.
Insert the swab into the extraction tube.
Rotate the swab at least 6 times.

Add 4 drops of sample solution to the well of the test cassette

Read the result within 15 minutes
How is the test performed?

Use the sterile nasopharyngeal swab provided in the kit, and carefully insert the swab into the patient’s nostril.

Add approximately 10 drops of the extraction buffer to the extraction tube.

Insert the swab into the extraction tube.
Rotate the swab at least 6 times.

Add 4 drops of sample solution to the well of the test cassette

Read the result within 15 minutes
Interpretation of results
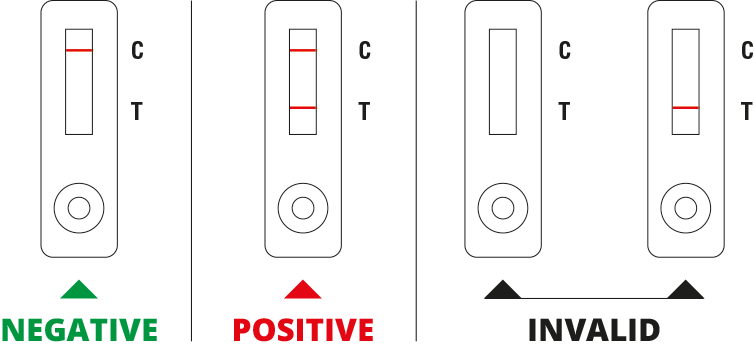
NEGATIVE: The presence of the control line (C) only within the result window indicates a negative result.
POSITIVE: The presence of two lines such as control line (C) and test line (T) within the result window indicates a positive result.
INVALID: If the control line (C) is not visible within the result window the result is considered invalid. Repeat the test.
